 |
 |
 |
|
RAYCHAUDHURI LAB @ CSIR-CCMB, Hyderabad |
 |
 |
 |
|
|
|
 |
 |
 |
|
|
|
|
|
|
|
|
| |
Inside a cell, numerous proteins interact with each-other to form a 'society'; the so-called 'cellular proteome'. Such 'protein-societies' are responsible for proper functioning of every cell. Any deviation from the functional conformation/concentration of a 'single member-protein' may negatively affect the organization of the 'society' and lead to gradual functional impairment. This is called 'proteotoxic-collapse' and is often true for many age-related diseases. We are a group of researchers @ CSIR-CCMB, Hyderabad interested in understanding the coordination of the proteins towards a functional proteome and the defense-mechanisms in the face of proteotoxic events.
Proteostatic control on protein-complex biogenesis
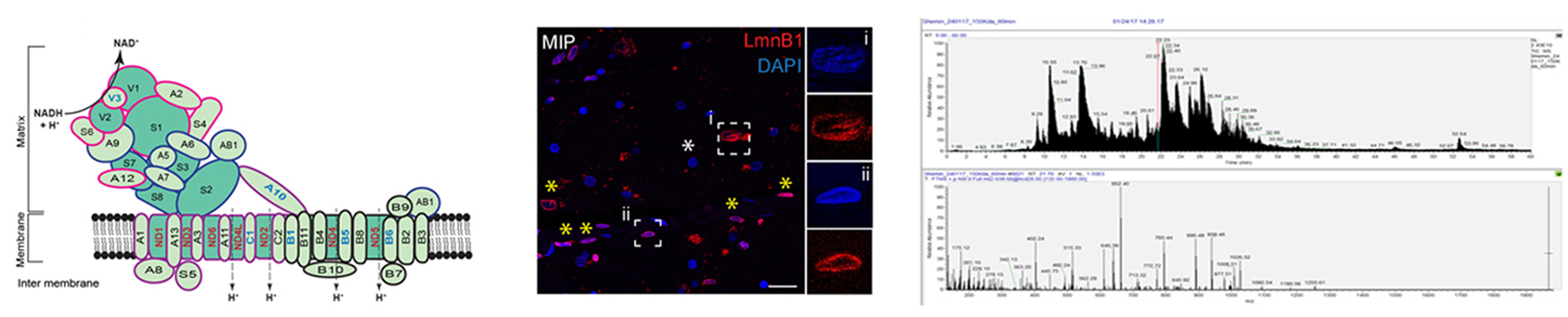
Respiratory complexes are multi-subunit protein complexes with the central function in aerobic energy metabolism. Earlier, we found that the protein subunits of respiratory complexes are aggregation-prone. We also observed that individual complexes associate and engage in higher-order supercomplexes to protect cellular respiration during stress. We hypothesize that aggregation propensities of respiratory complex subunits represent an adoptive evolution to facilitate their supra-organization in response to variety of stress conditions experienced in modern eukaryotic life. This complex organization faces proteostatic challenges during aging and contribute to pathologies of many age-related neurodegenerative diseases including Parkinsonís disease, Alzheimerís Disease, Amyotrophic Lateral Sclerosis (ALS) etc.
Cellular response to protein aggregation
We also work with amyloid inclusions. Amyloidogenic proteins form fibrillar inclusion bodies which are hallmark of many age-related neurological diseases. The pathological role of amyloid fibrils has obscure views, from being the cause of the pathogenesis to being the inert-end products of protein-misfolding. We work with a-Synuclein which is an amyloidogenic protein associated with Parkinsonís Disease (PD). The canonical pathological inclusions of a-Synuclein in PD are known as Lewy Bodies (LBs). We created models in primary neurons and cultured cells recapitulating LBs. Our results suggest that LBs are dump-yard of amyloidogenic a-Synuclein for eventual degradation. However, overgrown LBs deregulate cytoskeleton-nucleoskeleton connections at the perinucleus and damage the nuclear lamina. We further find that perturbed nuclear lamina is directly associated with deregulated transcription of stress-chaperones in LB-containing aging neurons.
Major focus areas
- Structural and functional evolution of Respiratory Complexes
- Membrane proteins and stress signalling
- Spatiotemporal quality control of amyloid inclusions
Research collaboration
- Dr. Koyeli Mapa, Shiv Nadar University
- Dr. Giriraj Chandak, CSIR-CCMB
- Dr. Shantanu Sengupta, CSIR-IGIB
- Dr. Dipyaman Ganguly, CSIR-IICB
- Dr. Abhishek Santra, CSIR-IICT
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Copyright (C) 2014, Swasti Raychaudhuri. All rights reserved. |
 |
 |
 |
 |
|